Research
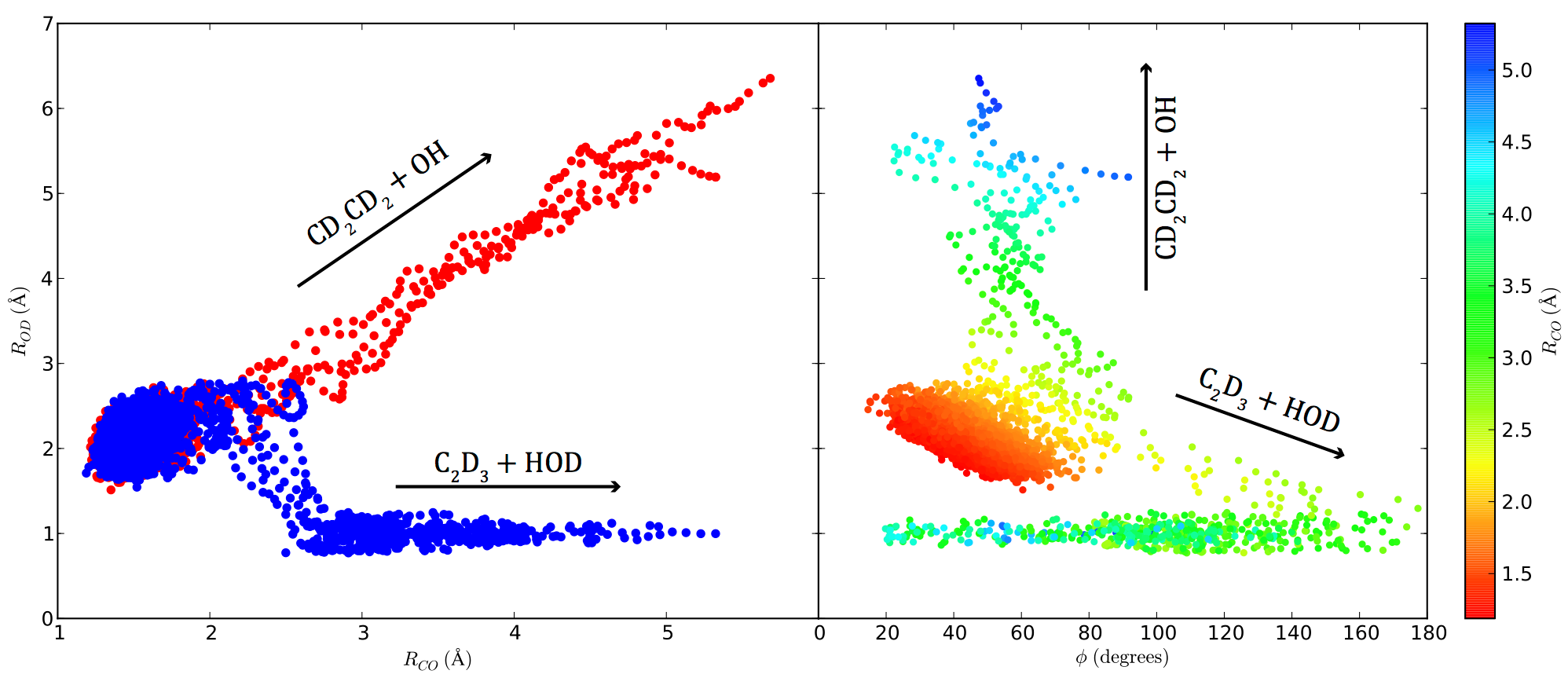
Our research uses experimental and theoretical methods to investigate the fundamental inter- and intramolecular forces that drive the course of chemical reactions. To experimentally probe the detailed molecular dynamics, both nuclear and electronic, during a chemical reaction we use a combination of molecular beam reactive scattering and laser spectroscopic techniques. Traditionally, predicting rate constants and microscopic dynamics has relied on statistical transition state theories or, in smaller systems, quantum scattering calculations on a single adiabatic potential energy surface that provides the barriers to each reaction. However, a reaction evolves on a single potential energy surface only if the Born-Oppenheimer separation of nuclear and electronic motion is valid. Some of our work investigates classes of important chemical reactions where the breakdown of the Born-Oppenheimer approximation near the transition state alters the dynamics and markedly reduces the reaction rate. [Review article] In other studies, we investigate bimolecular reactions by selectively producing each of the radical intermediates along the bimolecular reaction coordinate and probing the branching to product channels from each radical intermediate. See the publications page for important applications to atmospheric and combustion chemistry.